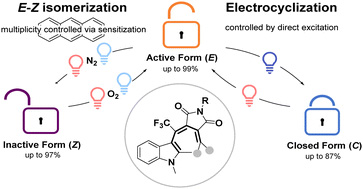
About our group
The research of our laboratory focuses on the development of small organic molecules that undergo electron transfer and/or can be activated by light. This interdisciplinary research field combines organic synthesis, electrochemistry, spectroscopy, physical chemistry and mechanistic investigations of light-triggered processes. Our main goal is to precisely control redox reactions and electron transfer in space and time, reversibly transfer charge between defined redox centres and develop methods for stabilization of organic radicals and radical ions. We aim to use them in various applications ranging from redox sensors to functionalized surfaces, molecular electronic devices and smart materials.
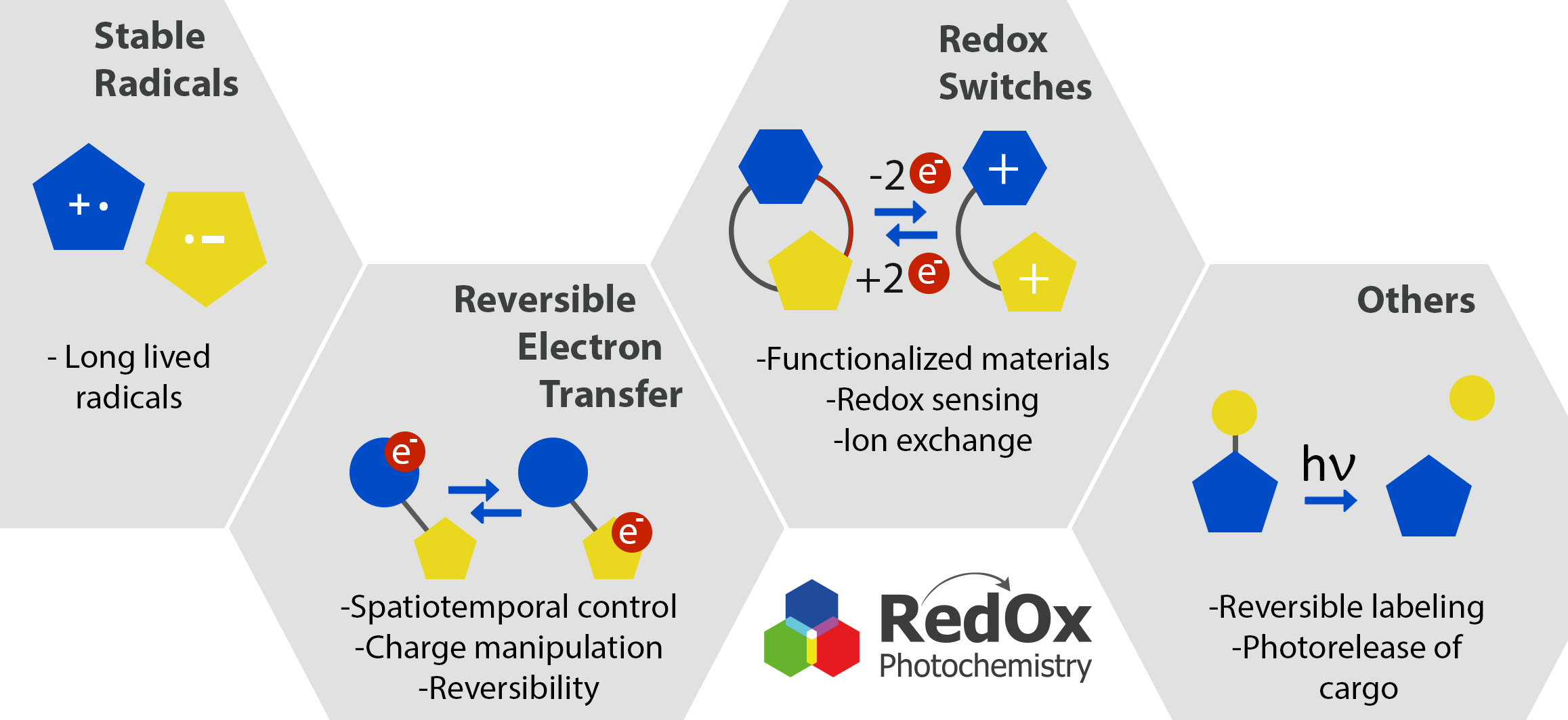
Publications
All publications