
Prof. Tamotsu Takahashi (Hokkaido University, Sapporo, Japan)
Carbon-Carbon Bond Cleavage and Formation on Titanocene
Abstract
Carbon-carbon bond cleavage is a challenging target in organic chemistry area. Titanacyclopentadiene can be conveniently prepared from Cp2TiCl2, 2 equiv of BuLi and two alkynes in high yields.1 Thus prepared titanacyclopentadiene 1 was treated with benzonitrile at 50 °C for 12h, tetraethylbenzene and diphenylpyridine were obtained in 50% and 38% yield, respectively. In this reaction, a cyclopentadienyl ligand was cleaved into a two carbon unit and a three carbon unit which were converted into benzene derivative and a pyridine derivative.2
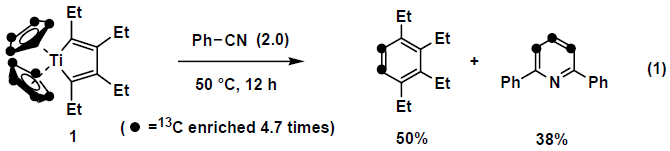
During our further study on this reaction, we found novel type of migration of an alkyl group from the diene moiety to the five membered ring moiety of indene derivative as shown below. In this reaction, cyclopentadienyl moiety was cleaved in the product. (eq (2))3
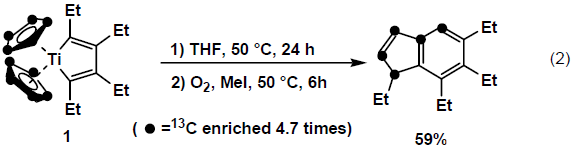
However, when 1 was treated with TiCl4, dihydroindene derivative was formed without cleavage of cyclopentadienyl moiety. (eq (3))4
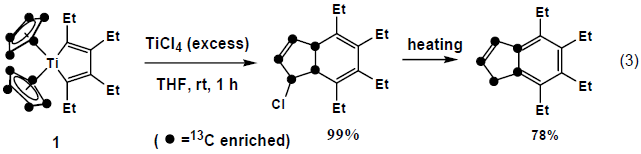
Acknowledgment: This project has been done as CREST and SORSRT projects of Japan Science and Technology Agency (JST).
References
- J. Organomet. Chem. 2001, 633, 18.
- J. Am. Chem. Soc. 2003, 125, 9568.
- J. Am. Chem. Soc. 2005, 127, 17188.
- J. Am. Chem. Soc. 2007, in press.








