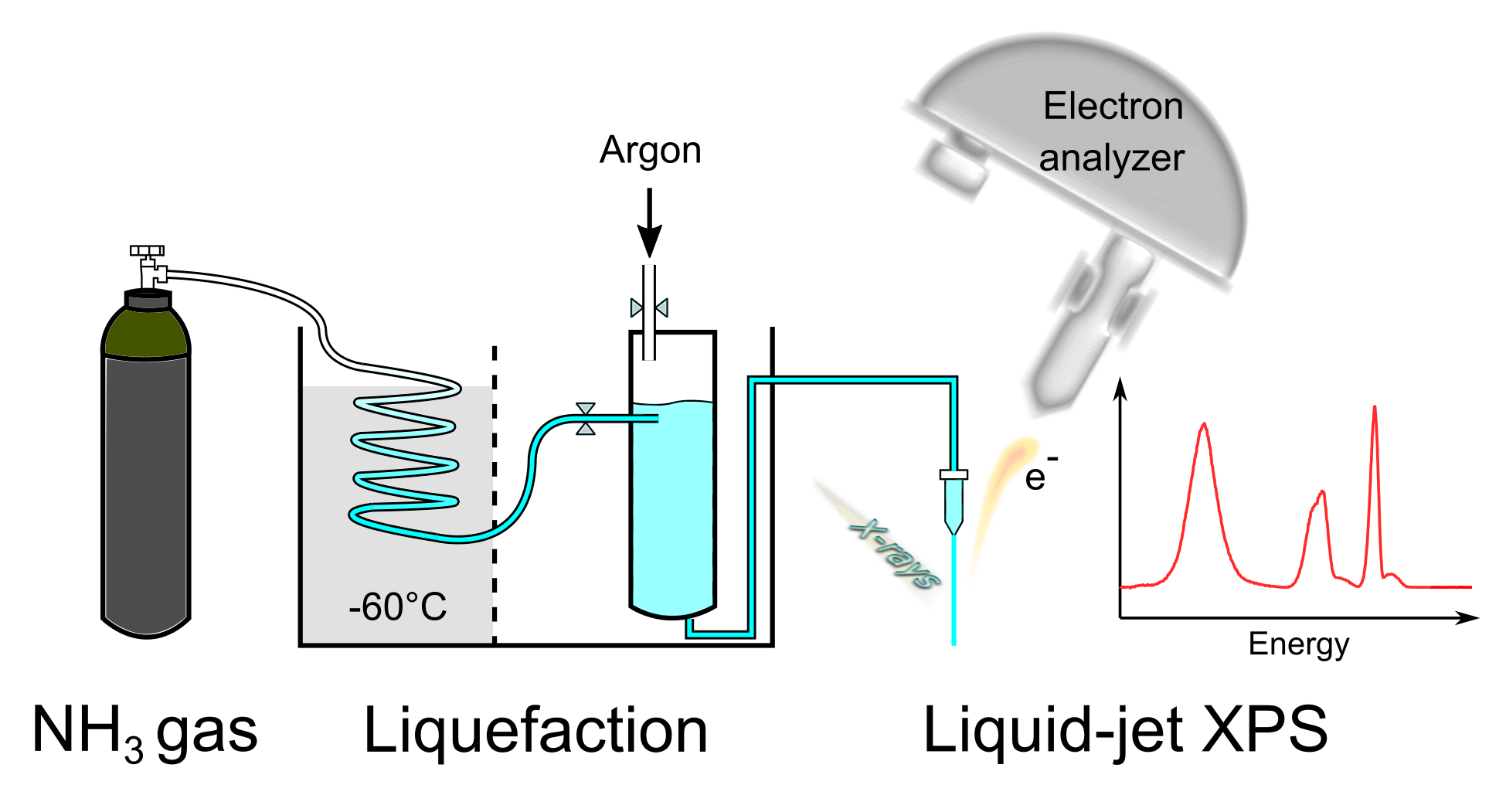
Originally, the X-ray photoelectron spectroscopy was established as a technique for the study of solid surfaces. With its help, it is possible to reveal the composition of the substance, the chemical and electronic state of the individual components, and it also allows to measure the kinetic energy and the number of electrons. More recently, it has been adapted for the study of liquids and transitions between liquid and gas phases by the introduction of so-called microjets. This method has already been used to study aqueous, acetonitrile, or alcoholic solutions.
Now, researchers from Pavel Jungwirth Group at IOCB Prague together with colleagues from University of Southern California (USA), Faculty of Mathematics and Physics, Charles University (Czech Republic), Helmholtz Center for Materials and Energy and Fritz Haber Institute of the Max Planck Society (Germany) used this method to study the properties of liquid as well as gaseous ammonia. This compound is commonly used e.g. as a solvent in organic chemistry, supporting the existence of long-lifetime (even several months) highly reducing solvated electrons in the liquid. Though it may seem that we know a lot about this simple four-atomic molecule, the international team has been able to reveal new interesting information reported as a Communication in the Journal of the American Chemical Society (JACS).
For the first time, X-ray photoelectron spectroscopy with microjets to vacuum was used to study the ammonia properties at low temperatures (-60 °C) and its transition from the liquid to the gaseous phase. The apparatus for the measurements was developed solely at IOCB with the actual experiments performed at the BESSY II synchrotron in Berlin.
Both valence and core bands of photoelectron spectra were measured. The differences between the liquid and gaseous phases of ammonia are mainly in photoelectron band shifts. The spectrum of the liquid phase allowed to clarify the way in which the hydrogen bonds in the condensed phase influence the electronic structure. Experiments were also supported by dynamical calculations based on ab-initio methods.
This research is important in predictions concerning the behavior and use of ammonia as a solvent that supports the long-lifetime highly-reducing solvated electrons. The results can also be used to study the reactions of other liquefied gases and thus to reveal more interesting information.
- Tillmann Buttersack, Philip E. Mason, Ryan S. McMullen, Tomas Martinek, Krystof Brezina, Dennis Hein, Hebatallah Ali, Claudia Kolbeck, Christian Schewe, Sebastian Malerz, Bernd Winter, Robert Seidel, Ondrej Marsalek, Pavel Jungwirth, Stephen E. Bradforth. Valence and Core-Level X-ray Photoelectron Spectroscopy of a Liquid Ammonia Microjet. Journal of the American Chemical Society 141 (5): 1838–1841, 2019.