Scientists from IOCB Prague are helping to better understand the mechanism of how genetic information is transcribed from DNA
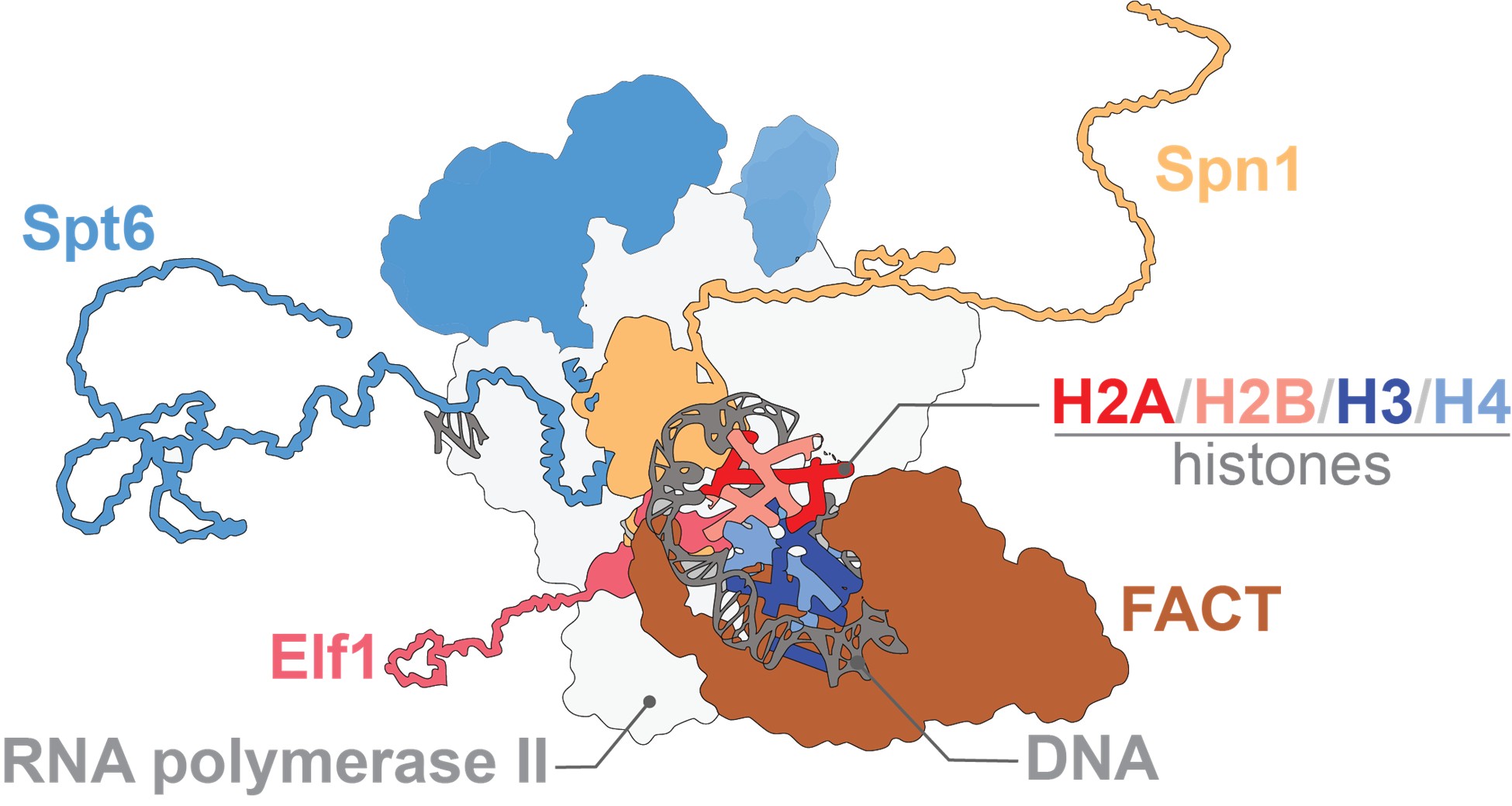
Our DNA is packaged as chromatin, tight coils of DNA wrapped around proteins called histones. For our genetic information to be read (transcribed from DNA into mRNA), these DNA coils must be temporarily loosened from the histones and then rebuilt with precision, an operation carried out by molecular “chaperones.” In a new study published in Molecular Cell, scientists reveal how one of the key proteins in this process, Spt6, cooperates with other chaperones to keep transcription running smoothly.

The research was the result of a close collaboration between Fred Winston’s team at Harvard Medical School and Václav Veverka’s group at IOCB Prague, with key contributions from James Warner and Vanda Lux. Together, they explored how chaperone Spt6 manages the delicate task of maintaining chromatin structure during gene transcription.
The scientists discovered that Spt6 relies on its amino-terminal domain, a flexible stretch of the protein, to hold onto the histones while the DNA is uncoiled and the genes are being transcribed. Without Spt6 function, cells cannot survive. Strikingly, the scientists also showed that when this domain of Spt6 is impaired, another chaperone, FACT, can step in to help. These discoveries led to a cooperative model in which different chaperones function in a coordinate fashion to ensure that the transcription machinery has constant access to DNA.
This study provides new insights on how Spt6 functions and helps to explain why cells depend on more than one histone chaperone. Rather than being redundant, these proteins dynamically support each other to maintain genome stability during transcription. Understanding gene transcription is important not only for basic research, but also for understanding human biology. Since problems in transcription and chromatin function are linked to various diseases, new insights into these mechanisms could eventually result in better treatments.
Original article
- Warner, J. L.; Lux, V.; Veverka, V.; Winston, F. The Histone Chaperone Spt6 Controls Chromatin Structure through Its Conserved N-Terminal Domain. Molecular Cell 2025, 85 (18), 3407-3424.e8. https://doi.org/10.1016/j.molcel.2025.08.020