Enzymatically stable galectin inhibitors
PI: Radek Pohl, co-PIs: Kamil Parkan, Jakub Kaminský, Petr Pachl, Marcela Pávová, Miroslav Hájek
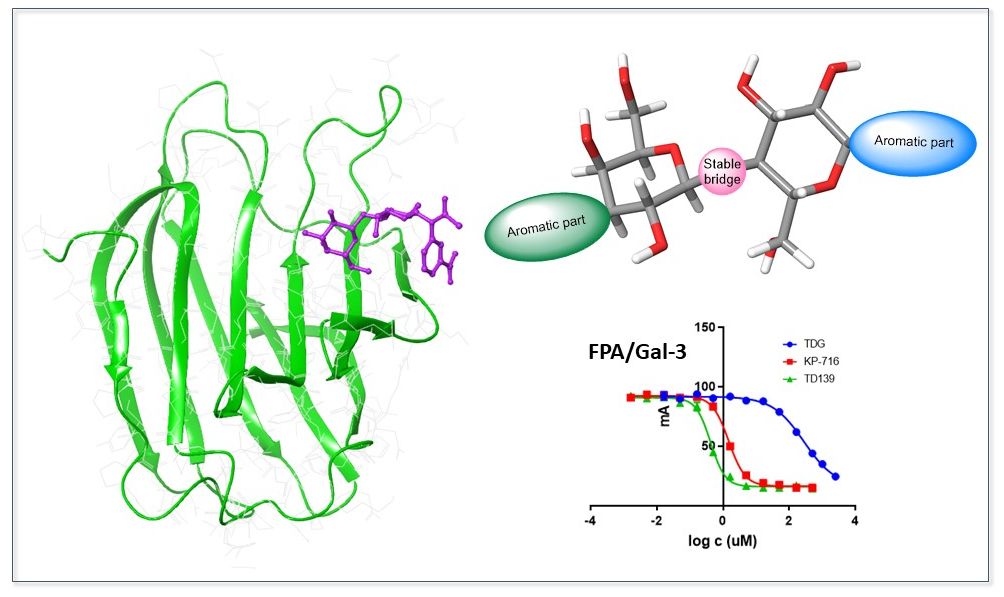
This interdisciplinary project deals with the design, synthesis and structure-activity relationship study of glycomimetics that bind to pharmaceutically relevant galectins, especially galectin-1 and galectin-3. These glycomimetics are structurally derived from galactose, lactose and thiodigalactoside carbohydrates. Special attention is paid to their chemical and enzymatic stability. Therefore, we develop a synthetic method enabling stereoselective preparation of alpha- and beta-anomers of C-, N- and S-glycosides and their disaccharide analogues. We also evaluate binding affinities to galectins by fluorescence polarization assay or using isothermal titration calorimetry and performance of our ligands in antiviral and anticancer cell-based assays. We study the structure of complexes of prepared ligands with galectins by X-ray crystallography that helps us together with molecular modelling to design new ligands with desired properties.
The GSRC-III program and support from Gilead Sciences have helped to put together people from all branches of science cultivated at IOCB (CHEM, BIO, PHYS) and thus initiated glycomimetics portfolio expansion that might be attractive in drug discovery programs of both IOCB and Gilead Sciences.



