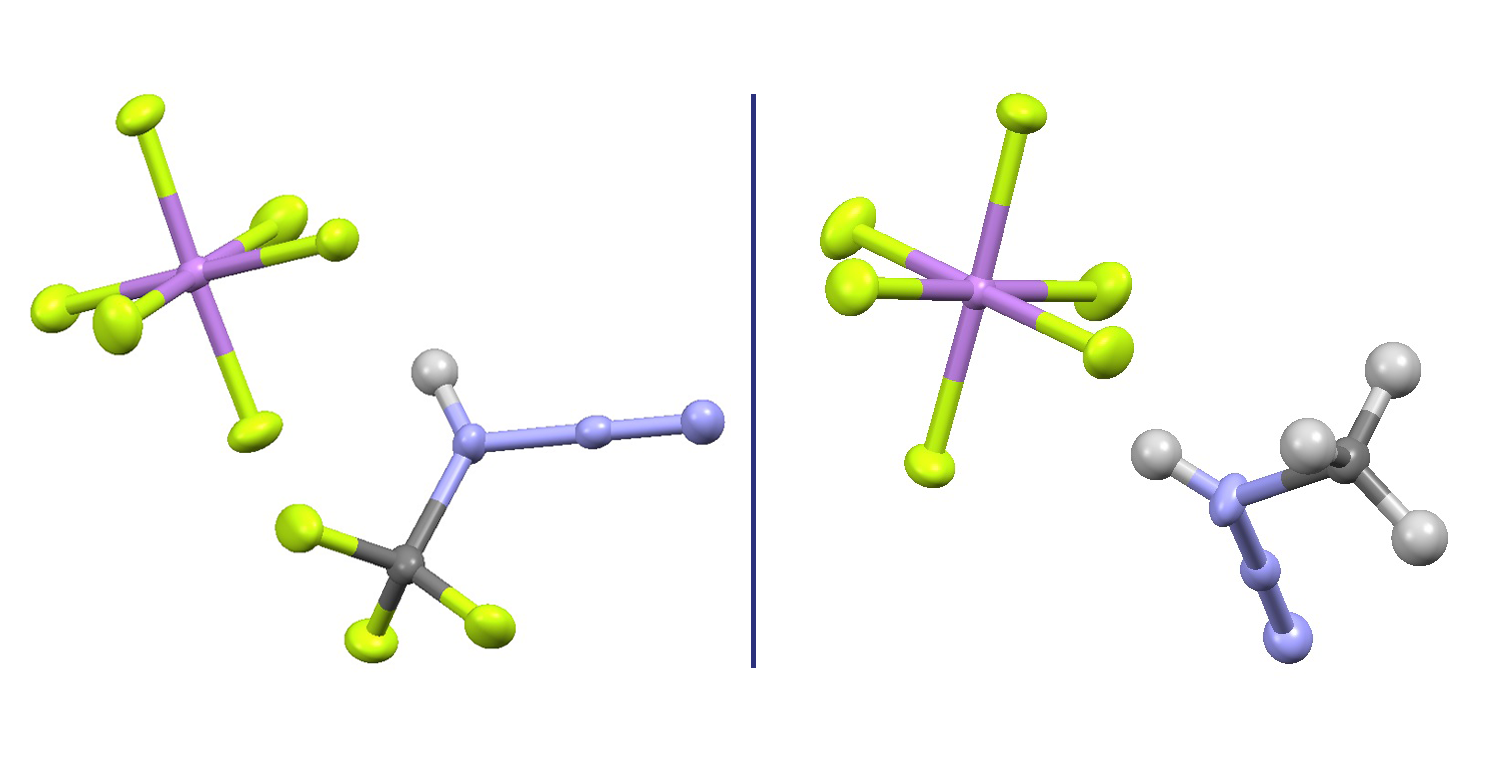
Zsófia Blastik and Petr Beier of IOCB Prague collaborated with scientists from the University of Southern California and the University of Alabama to discover the nature of protonated methyl- and trifluoromethyl azides, chemical species considered key intermediates in multiple reactions significant in the field of organic synthesis.
The team utilized superacidic conditions and cryogenic temperatures to prepare the azide salts in a crystalline form allowing for X-ray structure determinations. They also characterized the prepared compounds using multi-nuclear NMR and Raman spectroscopy, proving that the protonation site is on the carbon-bound nitrogen atom both in solution and in crystal.
To achieve this success, the researchers had to perform the key reaction stepwise in order to avoid the explosions that commonly occur when dealing with low-molecular organic azides. They also prepared azide∙AsF5 Lewis adducts and characterized them in a similar fashion.
These findings, published in Angewandte Chemie International Edition, give the researchers unprecedented insight into the nature of significant unstable intermediates and allow for finer determination of reaction mechanisms, thus paving the way for even more discoveries in azide chemistry and related nitrene reactions.
Original paper: Prakash, S..G., Saal, T., Blastik, Z..E., Haiges, R., Nirmalchandar, A., Baxter, A..F., Christe, K..O., Vasiliu, M., Dixon, D..A. and Beier, P. (2020), Protonation of CH3N3 and CF3N3 in Superacids: Isolation and Structural Characterization of Long‐Lived Methyl‐ and Trifluoromethylamino Diazonium Ions. Angew. Chem. Int. Ed. Accepted Author Manuscript. https://doi.org/10.1002/anie.202002750