Compounds of oxygen and iron in the 4+ oxidation state, called iron-oxo complexes, play an important role in oxidation reactions, such as the conversion of substances in the liver. These reactions often involve breaking of nonpolar C–H bonds, which forms radicals, i.e. molecules with unpaired electrons. Thus, the most reactive complexes are usually those with the greatest number of unpaired electrons. No iron-oxo complexes devoid of unpaired electrons were known due to the energetic instability of such an electronic configuration.
Erik Andris and Lubomír Rulíšek of IOCB Prague and Jana Roithová of Radboud University wanted to know if iron-oxo complexes without unpaired electrons could be prepared by using a systematic approach to ligand design.
Because they needed to screen a large number of molecules potentially capable of stabilizing this electronic state, and because synthesizing so many different molecules is not practical, they decided to predict their properties using a computational method based on the density-functional theory (DFT).
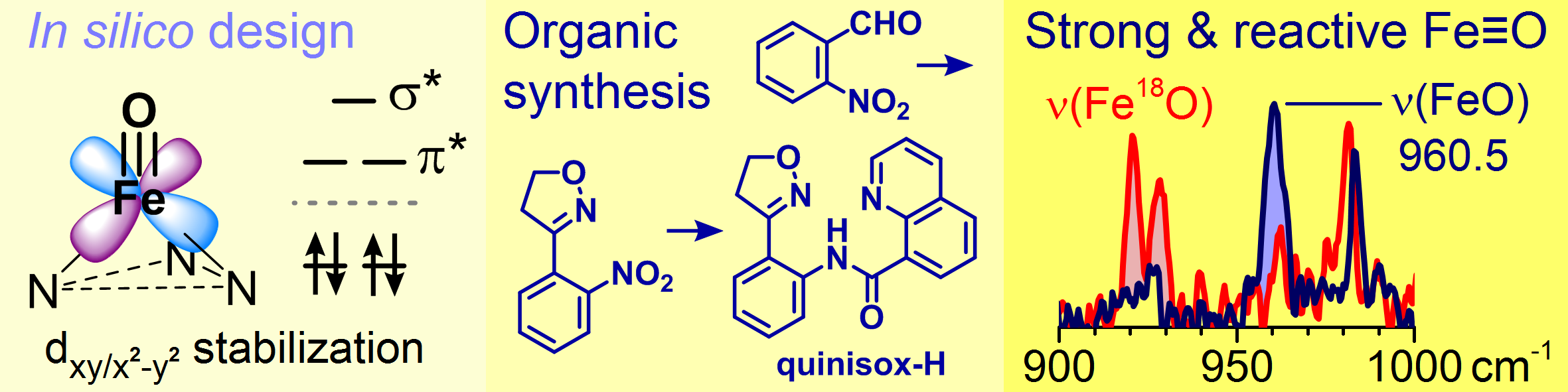
After many calculations, they selected several molecules with potential for the desired properties and subsequently synthesized them. Using visible and infrared photodissociation spectroscopy, which can be applied in mass spectrometry to ions in the gas phase, they verified that one of the predicted molecules did indeed possess the required properties. It was then determined that the prepared compound contained an as-yet-unobserved triple bond between the iron and oxygen. Even though strong bonds are typically nonreactive, contrary to expectation the researchers observed that their molecule possesses a high degree of reactivity and that the necessary radical centers form during the reaction.
These findings demonstrate that it is possible to use theoretical chemistry methods to discover molecules with elusive electronic states. Thanks to this, the researchers proved the existence of a new type of iron-oxo compounds called singlet iron-oxo organic complexes. The designed ligands, which are capable of stabilizing this unusual phenomenon, may also find application in other chemical reactions with different transition metals.
The original article
E. Andris, K. Segers, J. Mehara, L. Rulíšek, J. Roithová, Closed Shell Iron(IV) Oxo Complex with an Fe–O Triple Bond: Computational Design, Synthesis, and Reactivity. Angew. Chem. Int. Ed. 2020, 59, 23137. https://doi.org/10.1002/anie.202009347