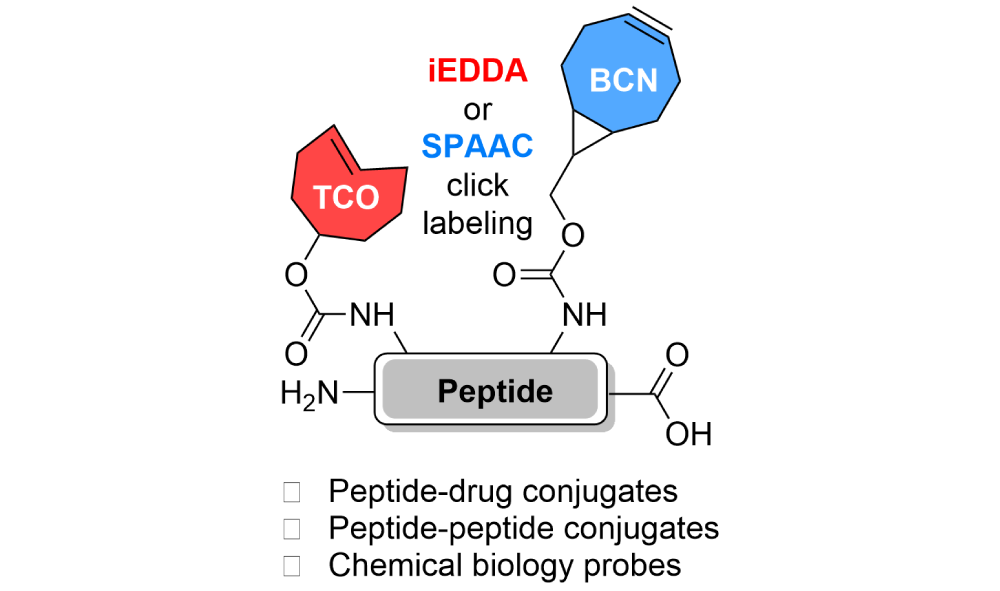
Peptides are a unique class of biomacromolecules combining the advantages of small molecules and larger biomolecules. Synthetic peptides are produced by solid-phase peptide synthesis (SPPS), which also enables the incorporation of non-canonical or modified amino acids. Unfortunately, the introduction of highly reactive groups suitable for bioorthogonal reactions, such as dienophilic trans-cyclooctene (TCO) and bicyclononyne (BCN), remained elusive until now.
The scientists led by Milan Vrábel from IOCB Prague have developed an SPPS protocol enabling the introduction of TCO- and/or BCN-derivatives into the peptide chain. The strategy is based on the combination of post-synthetic derivatization using TCO- and BCN- active esters and selective deprotection steps.
The scientists showed that the modified peptides could be easily used for the construction of peptide-drug or peptide-peptide conjugates and could serve as multifunctional chemical biology probes.
The synthetic strategy and application examples have been published in Chemistry–A European Journal as a hot paper with Agustina La-Venia as the first author.
The original paper:
- La–Venia, A., Dzijak, R., Rampmaier, R. and Vrabel, M. (2021), An Optimized Protocol for the Synthesis of Peptides Containing trans-Cyclooctene and Bicyclononyne Dienophiles as Useful Multifunctional Bioorthogonal Probes. Chem. Eur. J. https://doi.org/10.1002/chem.202102042