Nucleosides are the foundation of life, but are also implicated in disease and medicine chiefly as antiviral or anticancer drugs. Unfortunately, the production of both synthetic and natural nucleosides is usually a laborious and tedious process, which often lacks efficiency or selectivity (leading to mixtures of products) and can involve the use of highly toxic reagents. Therefore, improved methods to synthesize these crucial molecules are important to several fields of study and could be of medicinal value.
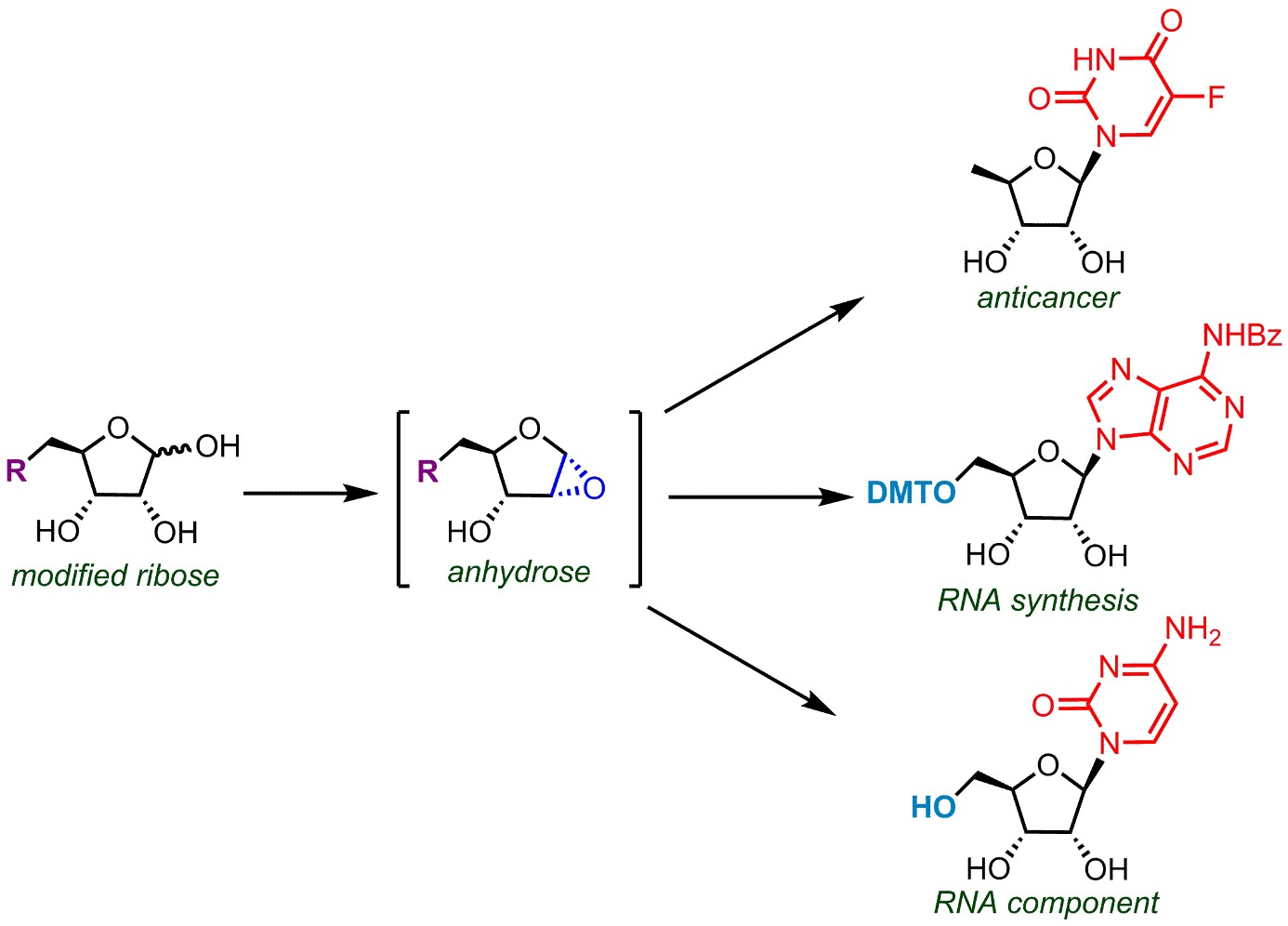
A. Michael Downey, a PhD student from Canada, with his supervisor Prof. Michal Hocek and collaborators at the Institute of Organic Chemistry and Biochemistry (IOCB Prague) and Charles University designed and developed a new simple and selective method to synthesize these extremely valuable molecules by direct glycosylation of nucleobases with modified ribose via a key novel intermediate. The procedure consists of only one single operation (two-step one-pot procedure) and uses non-toxic reagents.
Another advantage is the stereoselectivity of this reaction, which affords only the desired stereoisomer. This research provides an easier way to create adenosine, guanosine, uridine, and cytidine, the four canonical components of RNA. It also facilitates access to anticancer drugs (such as doxifluridine), biologically interesting molecules (5′-deoxy-5′-fluoroadenosine) or building block for automated solid-phase synthesis of oligonucleotides (5'-dimethoxytritylated nucleosides).
The paper has been published in Chemistry – A European Journal:
- Downey, A. M.; Pohl, R.; Roithová, J.; Hocek, M. Synthesis of nucleosides through direct glycosylation of nucleobases with 5-O-mono-protected or 5-modified ribose. Improved protocol, scope and mechanism. Chem. Eur. J. 2017, DOI: 10.1002/chem.201604955.
Read next...