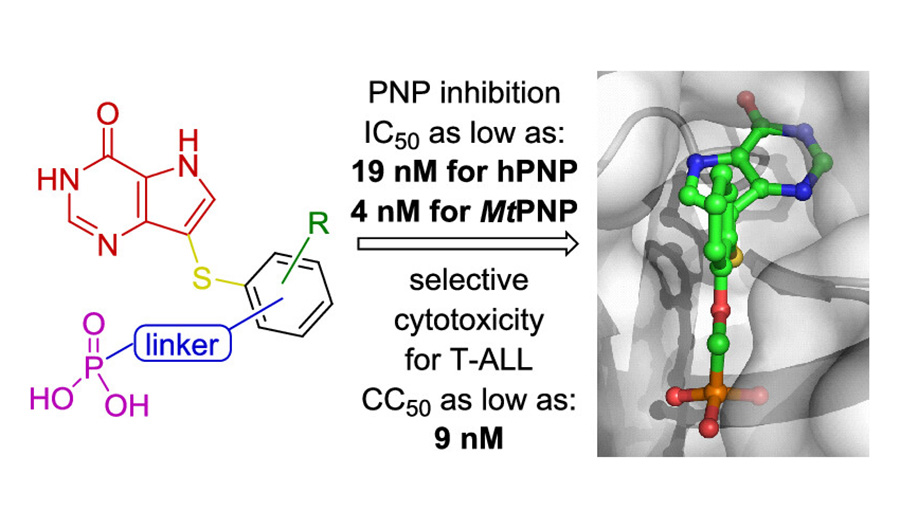
Purine nucleoside phosphorylase (PNP) is a well-known molecular target with potential therapeutic applications in the treatment of T-cell malignancies and various bacterial and parasitic infections. Three IOCB research groups led by Zlatko Janeba (Medicinal Chemistry of Nucleotide Analogues), Helena Mertlíková-Kaiserová (Biochemical Pharmacology), and Pavlína Maloy Řezáčová (Structural Biology) joined their efforts to prepare and study novel PNP inhibitors based on acyclic nucleoside phosphonates bearing a 9-deazahypoxanthine nucleobase.
The researchers (led by Jan Skácel) have developed an efficient synthetic methodology, which allowed them to combine structural features of previously reported potent PNP inhibitors. Such methods were not accessible by previously published synthetic approaches. They have prepared a series of 30 compounds which were screened for inhibitory activity against recombinant PNP from Homo sapiens, Mycobacterium tuberculosis, and Plasmodium falciparum and on several T-lymphoblastic cell lines and non-T-cell cancer cell lines.
The biological studies showed that the strongest inhibitors exhibited IC50 values as low as 19 nM for human PNP and 4 nM for Mycobacterium tuberculosis PNP and highly selective cytotoxicity toward various T-lymphoblastic cell lines with CC50 values as low as 9 nM. Researchers observed no cytotoxic effect on other cancer cell lines (HeLa S3, HL60, HepG2) or primary PBMCs for up to 10 μM. They also described the first example of the PNP inhibitor exhibiting over 60-fold selectivity for the pathogenic enzyme (MtPNP) over human PNP. The obtained data strongly suggest the feasibility of future development of potential selective PNP inhibitor-based therapeutics against various pathogens.
In addition to that, the researchers (main contribution by Stefan Djukic) conducted extensive crystallography study of eight enzyme-inhibitor complexes providing details on their activity and selectivity, as well as on flexibility of both PNP enzymes and the inhibitors. Selected compounds were subjected to in vitro ADME profiling and an in vivo pharmacokinetic study.
Read the paper: Skácel, J.; Djukic, S.; Baszczyňski, O.; Kalčic, F.; Bílek, T.; Chalupský, K.; Kozák, J.; Dvořáková, A.; Tloušt’ová, E.; Král’ová, Z.; Šmídková, M.; Voldřich, J.; Rumlová, M.; Pachl, P.; Brynda, J.; Vučková, T.; Fábry, M.; Snášel, J.; Pichová, I.; Řezáčová, P.; Mertlíková-Kaiserová, H.; Janeba, Z. Design, Synthesis, Biological Evaluation, and Crystallographic Study of Novel Purine Nucleoside Phosphorylase Inhibitors. J. Med. Chem. 2023, 66, 6652-6681. https://doi.org/10.1021/acs.jmedchem.2c02097