
Most single bonds in molecules can twist and turn freely, but there are some with a special kind of bond that can't. These are called atropisomers, and their movement bears resemblance to a Japanese beckoning cat whose paw moves only up and down but never all the way around. Whether the cat can bring us luck or not is up for debate, but what is indisputable is that atropisomers have already brought – and will continue to bring – prosperity to the process of drug discovery and development. Atropisomers can behave in unique ways inside the body, making them promising candidates for designing more precise and effective medicines.
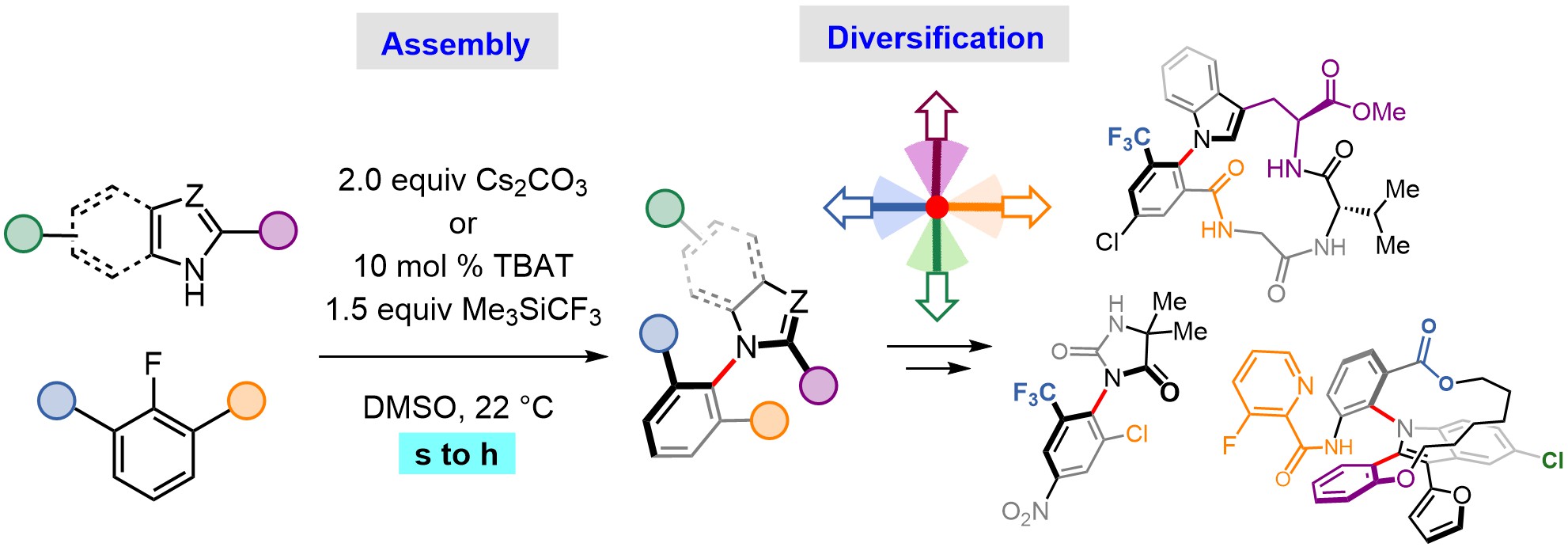
It is important to understand that, to restrict single bond motion, bulky groups need to be incorporated around it. Why does this matter? Atropisomers are inherently hindered, thus synthesizing them can easily become a nightmare. The most direct atropisomer formation strategies usually require high-energy pahtways, leading to harsh reaction conditions and hard-to-obtain starting materials. Many times, other parts of the molecule fall apart, leading to what chemists call a “lack of chemoselectivity”. These problems particularly apply to molecules which are more likely to become pharmaceuticals.
A team of organic chemists led by Paulo Paioti at IOCB Prague developed a platform to produce drug-like atropisomers with C–N restricted bonds. The processes are practical, inexpensive and can be carried out extremely quickly; all features that accelerate discovery of bioactive molecules. “Our work gave access to a broad variety of complex molecules that were previously out of reach. I was often surprised by how reactive and functional group tolerant the reactions were. Many times, not even purification was needed to obtain pure compounds,” says PhD student Paritosh Dey. Dr. Michal Šimek, who spearheaded the project in the lab, adds: “The most interesting thing is that we achieve all this with a textbook reaction that’s 150 years old! This is great because our chemistry is accessible and robust. It was somehow just severely underexplored for atropisomer production.”
The advances resulted from investigation of a class of reactions called nucleophilic aromatic substitution (SNAr). In collaboration with computational chemist Dr. Daniel Bím from UCT Prague, the team also provided evidence that SNAr reactions bypass the high-energy, sterically hindered intermediates that are common to atropisomer synthesis. The key takeaway of the mechanistic study is that the synthesis of hindered molecules doesn’t necessarily have to proceed via hindered pathways. This line of thought is to spark the development of more of such efficient processes.
The method represents a fast track to a novel and unique class of potentially therapeutic compounds and is of fundamental relevance to synthesis of challenging hindered single bonds.
Read the paper: Šimek, M.; Dey, P.; Blahout, V.; Mondal, K.; Ernenwein, J.; Dračínský, M.; Bím, D.; Paioti, P. H. S. Nucleophilic Aromatic Substitutions Enable Diversity-Oriented Synthesis of Heterocyclic Atropisomers via Non-Atropisomeric Intermediates. Nat. Commun. 2025, 16, 4856. https://doi.org/10.1038/s41467-025-60101-z





