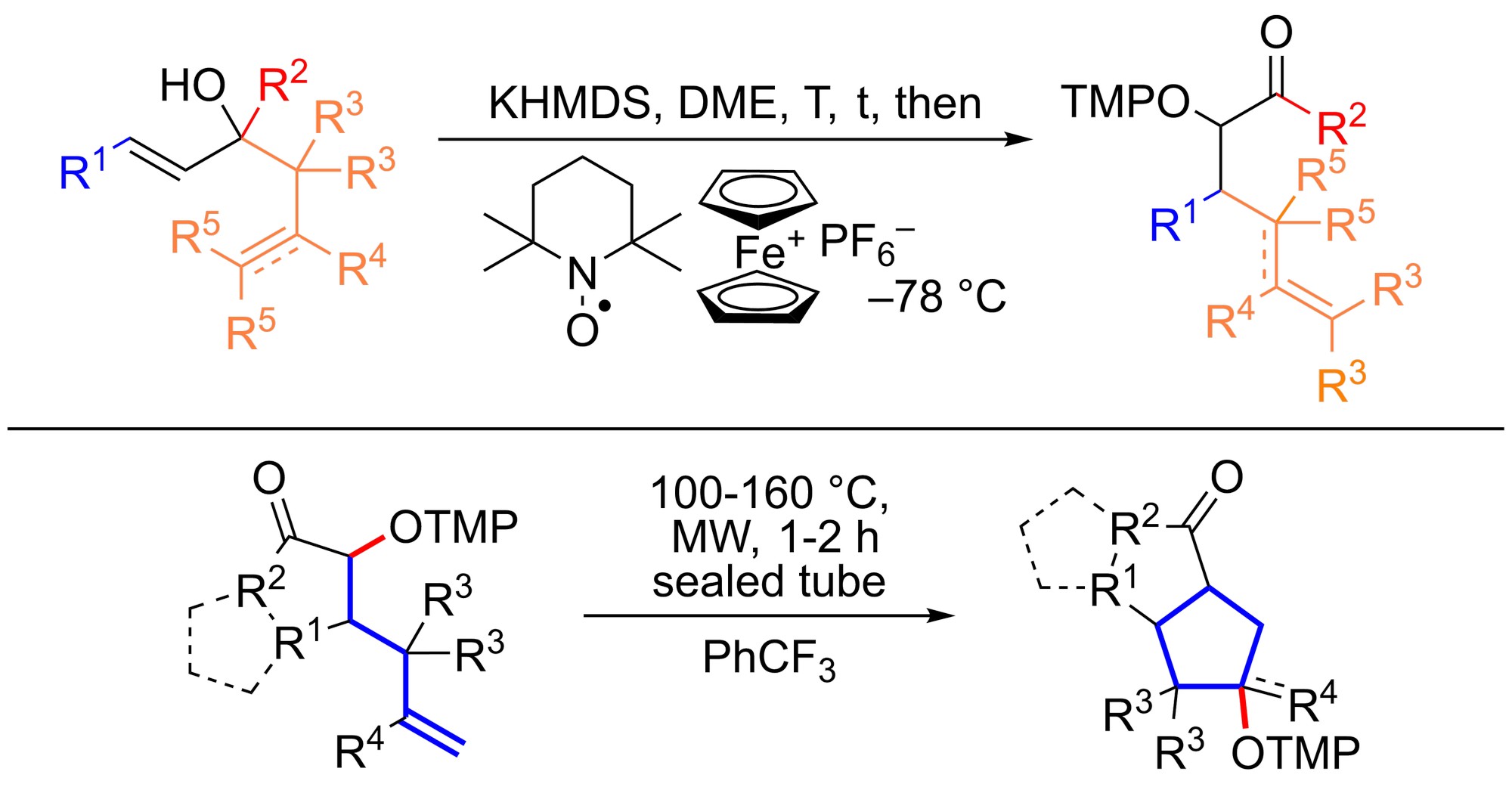
Michal Šimek and Ullrich Jahn show in their Angewandte Chemie paper how to rapidly construct functionalized carbocycles in atom economic steps enabled by a clever merger of pericyclic and radical reactions.
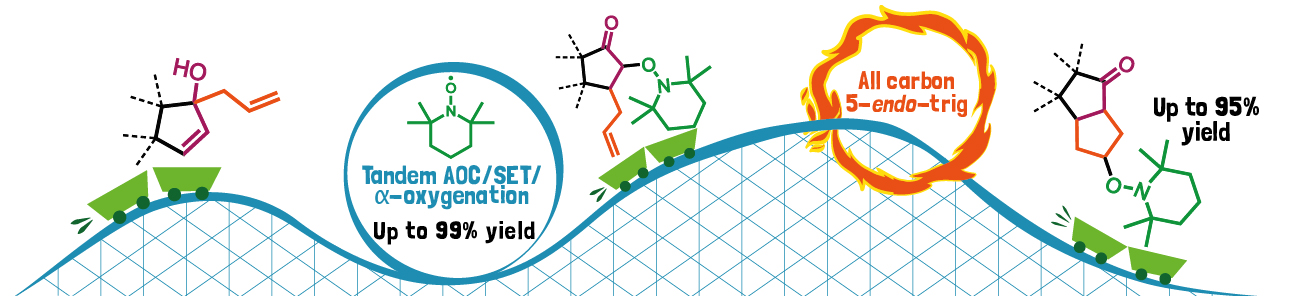
The authors present in their communication a reaction sequence that begins with a [3,3]-rearrangement, which modifies the carbon skeleton, puts a double bond where it needs to be for cyclization and installs an alkoxyamine handle at the same time. This unit is then relocated onto the double bond in a rare 5-endo-trig cyclization step, forming cyclopentanes with the alkoxyamine as a useful oxygen functionality for further transformations.
The overall process broadens the repertoire of tools for chemists in the synthesis of complex molecules and will serve as a blueprint for further development and applications for chemistry and biology.