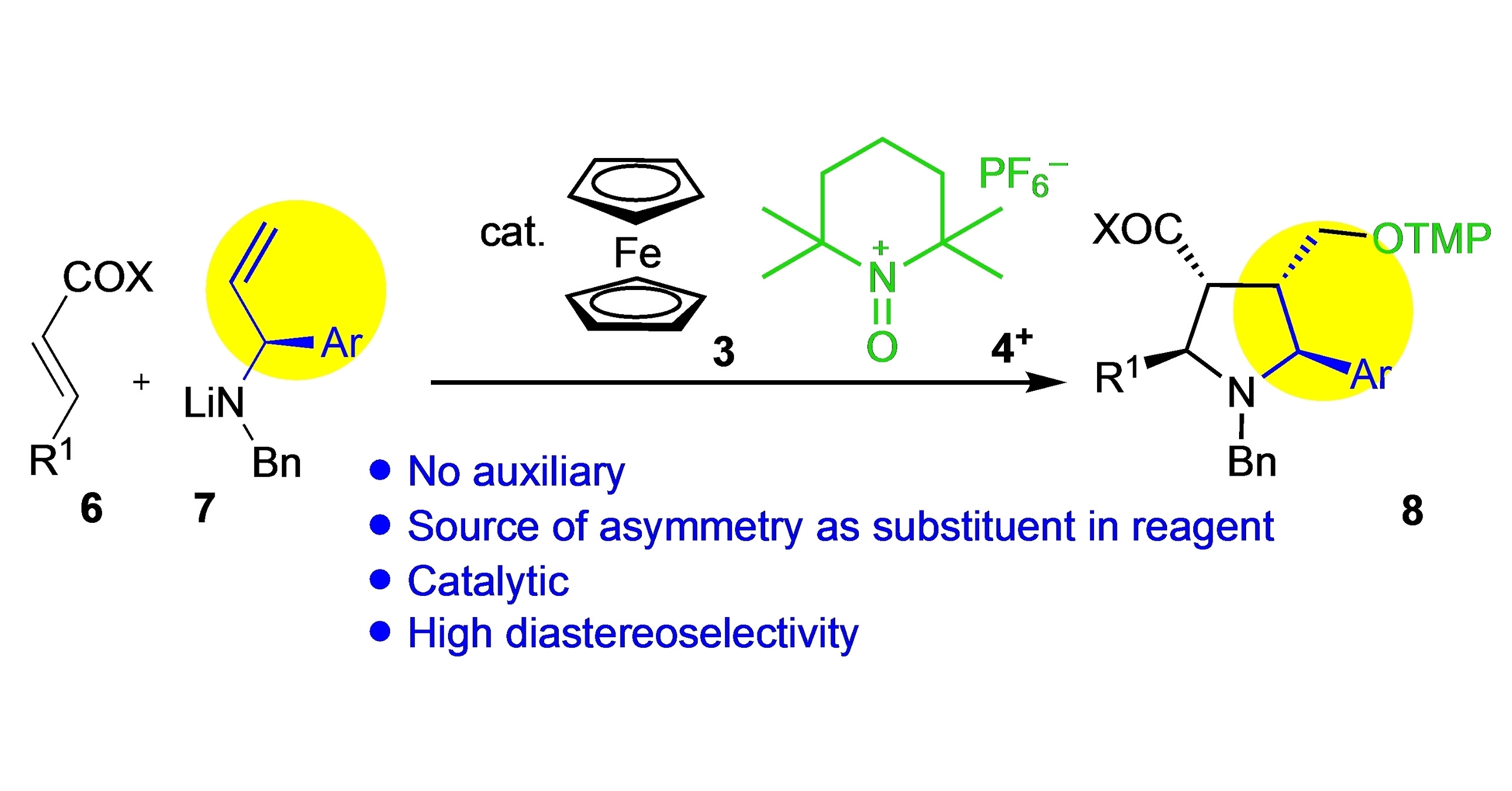
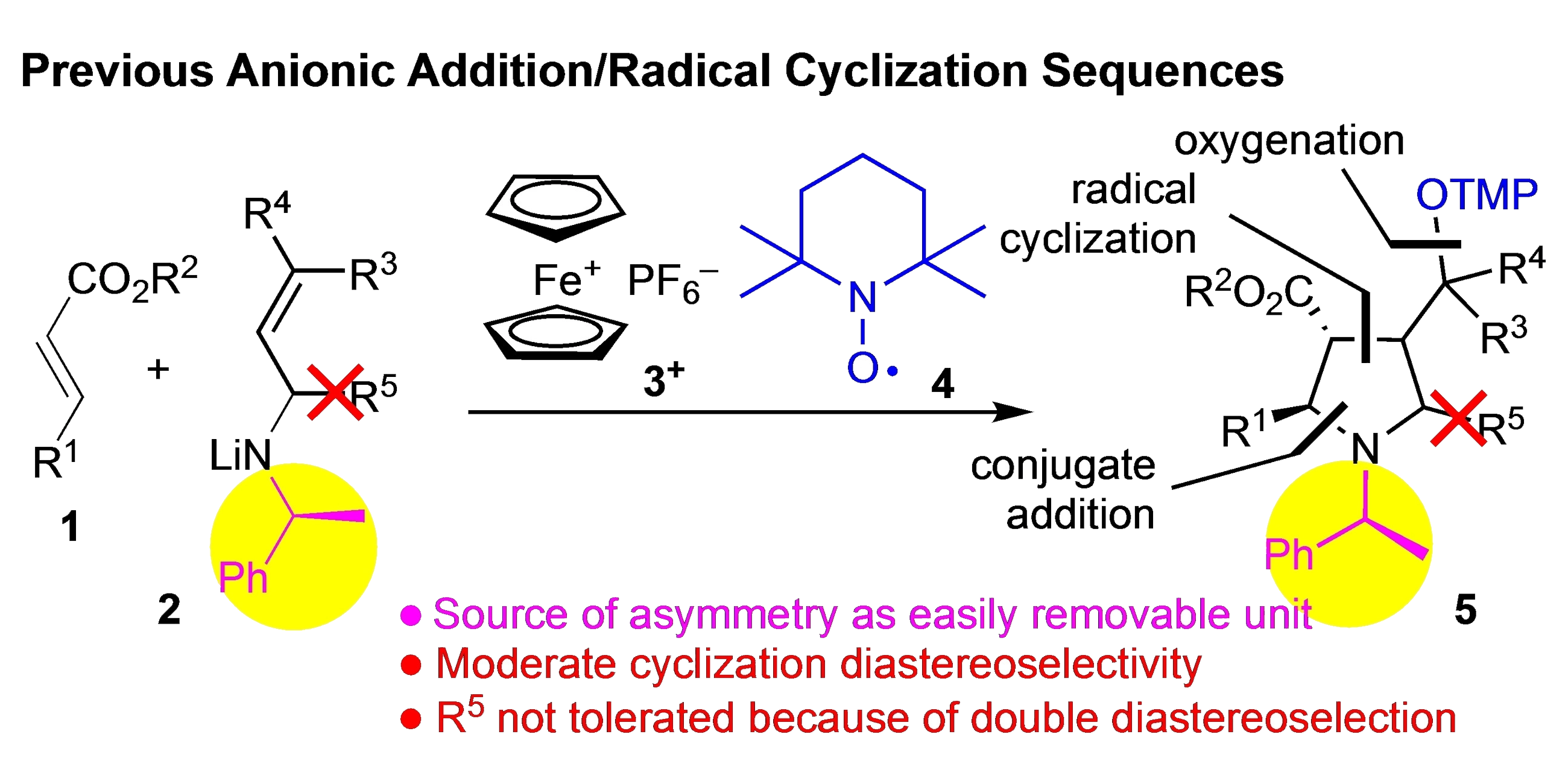
Pentasubstituted pyrrolidines are ubiquitous constituents of natural products and drugs, however, they are not readily accessible especially with respect to absolute and relative stereochemistry at the pyrrolidine ring.
Denisa Hidasová and colleagues led by Ullrich Jahn from IOCB Prague developed an asymmetric tandem synthetic approach leading to N,2,3,4,5-pentasubstituted pyrrolidines. Using enantiomerically enriched allylamines and β-substituted-α,β-unsaturated esters sets the configuration at four stereocenters in the single-step reaction.
In addition, the authors designed a model to predict the overall stereoselectivity. The methodology opens novel opportunities for the synthesis of biologically interesting N-heterocycles.
Read the paper:
Hidasová, D.; Pohl, R.; Císařová, I.; Jahn, U. A Diastereoselective Catalytic Approach to Pentasubstituted Pyrrolidines by Tandem Anionic-Radical Cross-Over Reactions, Adv. Synth. Catal. 2021, 363, 1-9. https://doi.org/10.1002/adsc.202101172