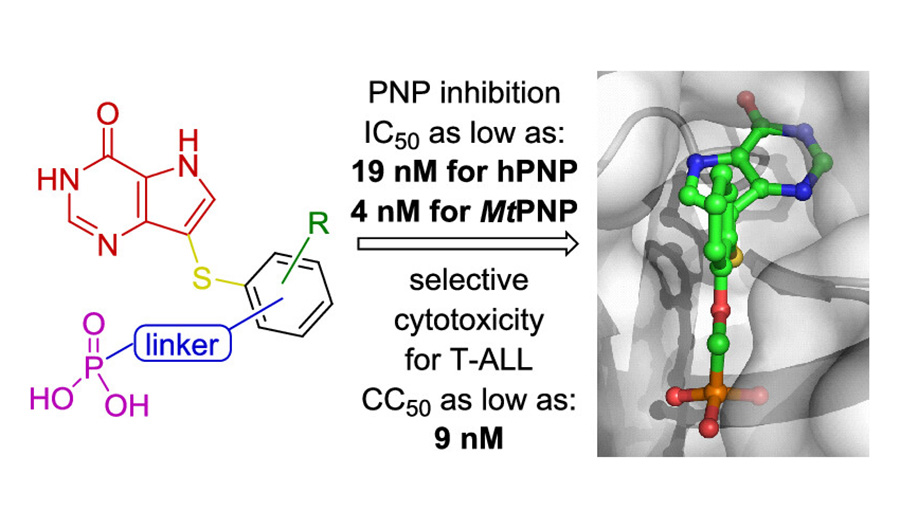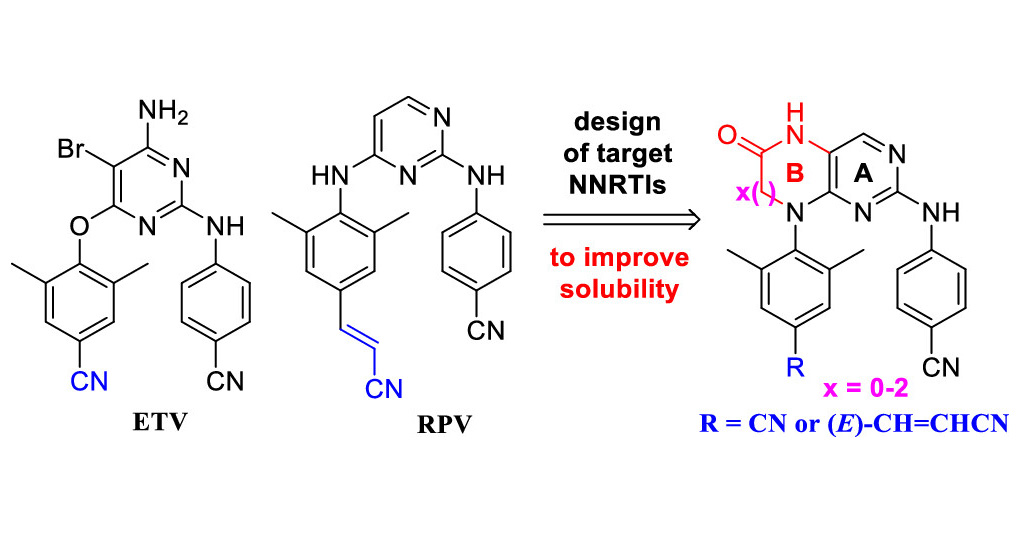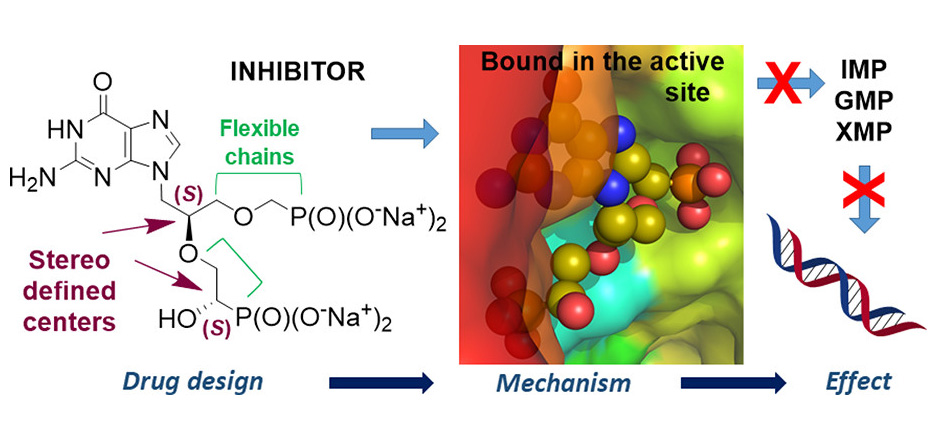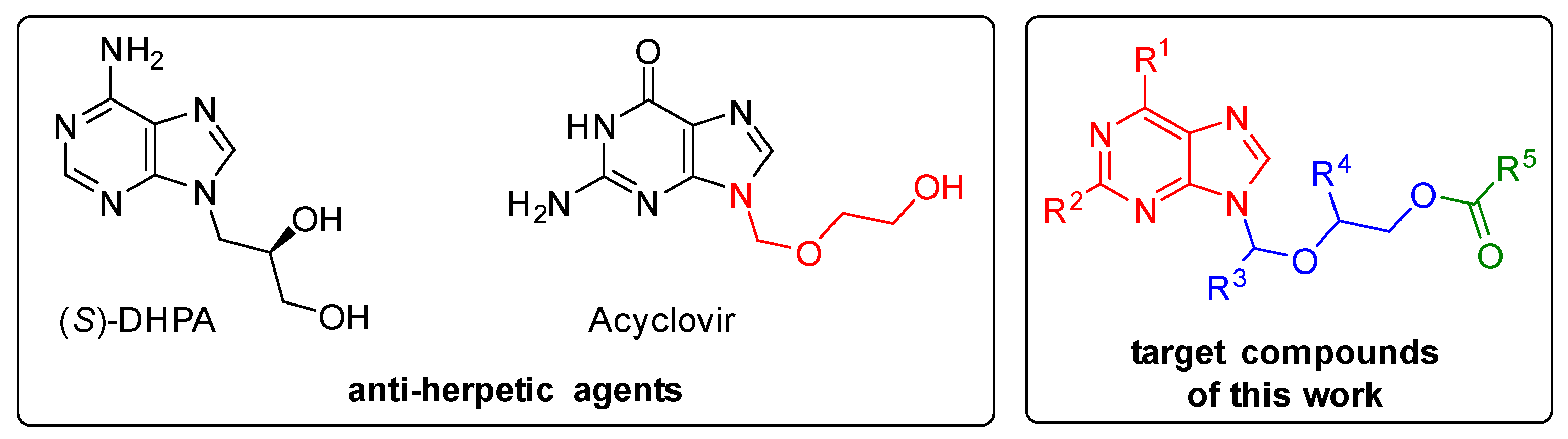
Jan Frydrych and researchers from IOCB Prague led by Zlatko Janeba reported a new efficient approach to the synthesis of acylated acyclic nucleosides containing a branched hemiaminal ether moiety via three-component alkylation of N-heterocycle with acetal and anhydride.
The procedure employs cheap and easily available acetals, acetic anhydride, and trimethylsilyl trifluoromethanesulfonate. It exhibits a broad substrate scope of N-heterocycles and acetals, and, in the case of purine derivatives, also excellent regioselectivity, giving almost exclusively N-9 isomers.
Read the paper:
- Frydrych, J.; Poštová Slavětínská, L.; Dračínský, M.; Janeba, Z. Efficient Synthesis of α-Branched Purine-Based Acyclic Nucleosides: Scopes and Limitations of the Method. Molecules 2020, 25, 4307. https://doi.org/10.3390/molecules25184307
Read next...