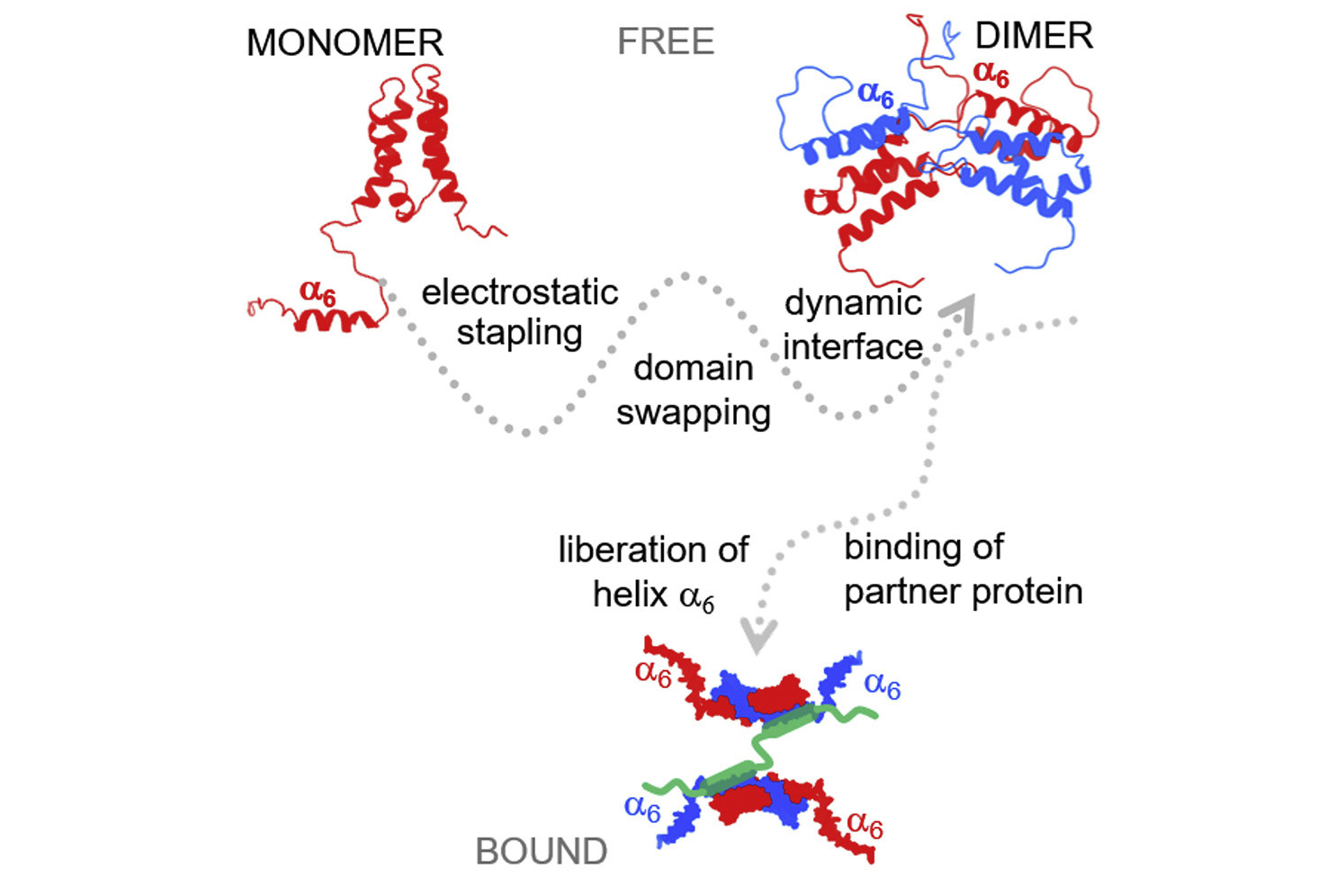
The dimerization is critical for the function of many eukaryotic transcription regulatory factors, including the epigenetic reader LEDGF/p75. Researchers led by Václav Veverka from IOCB Prague and Zeger Debyser from KU Leuven studied the minimal dimerization region in the C-terminal part of this protein and using paramagnetic NMR spectroscopy identified the key molecular contacts that helped to refine the solution structure of the dimer.
The researchers found out that LEDGF dimer is stabilized by domain swapping and additional electrostatic stapling and showed how dimerization might affect the LEDGF/p75 interactome.
Read the paper:
- Lux, V.; Brouns, T.; Čermáková, K.; Srb, P.; Fábry, M.; Mádlíková, M.; Hořejší, M.; Kukačka, Z.; Novák, P.; Kugler, M.; Brynda, J.; DeRijck, J.; Christ, F.; Debyser, Z.; Veverka, V. Molecular Mechanism of LEDGF/p75 Dimerization. Structure 2020. https://doi.org/10.1016/j.str.2020.08.012
Read next...