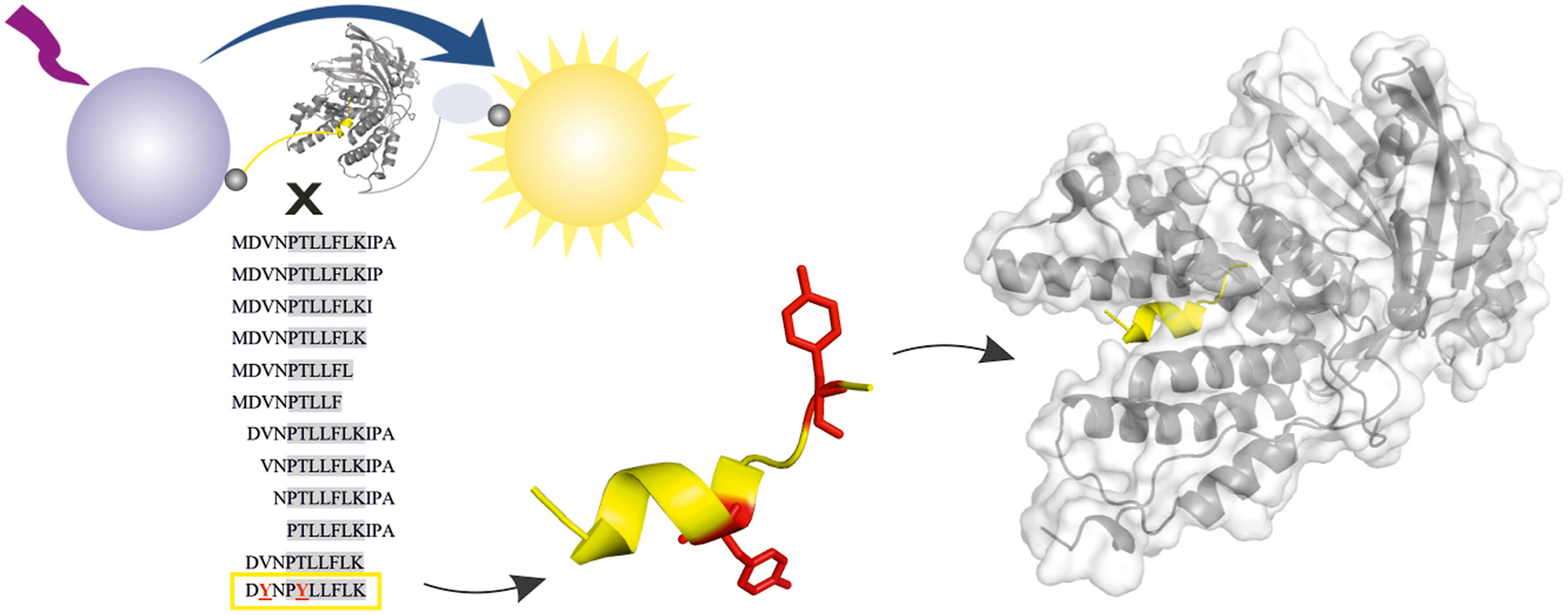
Current antivirotics target three key proteins in the life cycle of influenza virus: neuraminidase, the M2 channel, and the endonuclease domain of RNA-dependent-RNA polymerase. Due to the development of new strains, additional antiviral drugs targeting different viral proteins are still needed. And protein-protein interaction between polymerase subunits PA and PB1 is one such possible target.
The collaborative research work of PhD students Kateřina Radilová, Michal Kráľ, and Václav Zima from Jan Konvalinka and Pavel Majer groups at IOCB Prague, recently published in the Antiviral Research, describes the optimization of protein-protein interaction inhibitor that targets influenza RNA-dependent RNA polymerase.
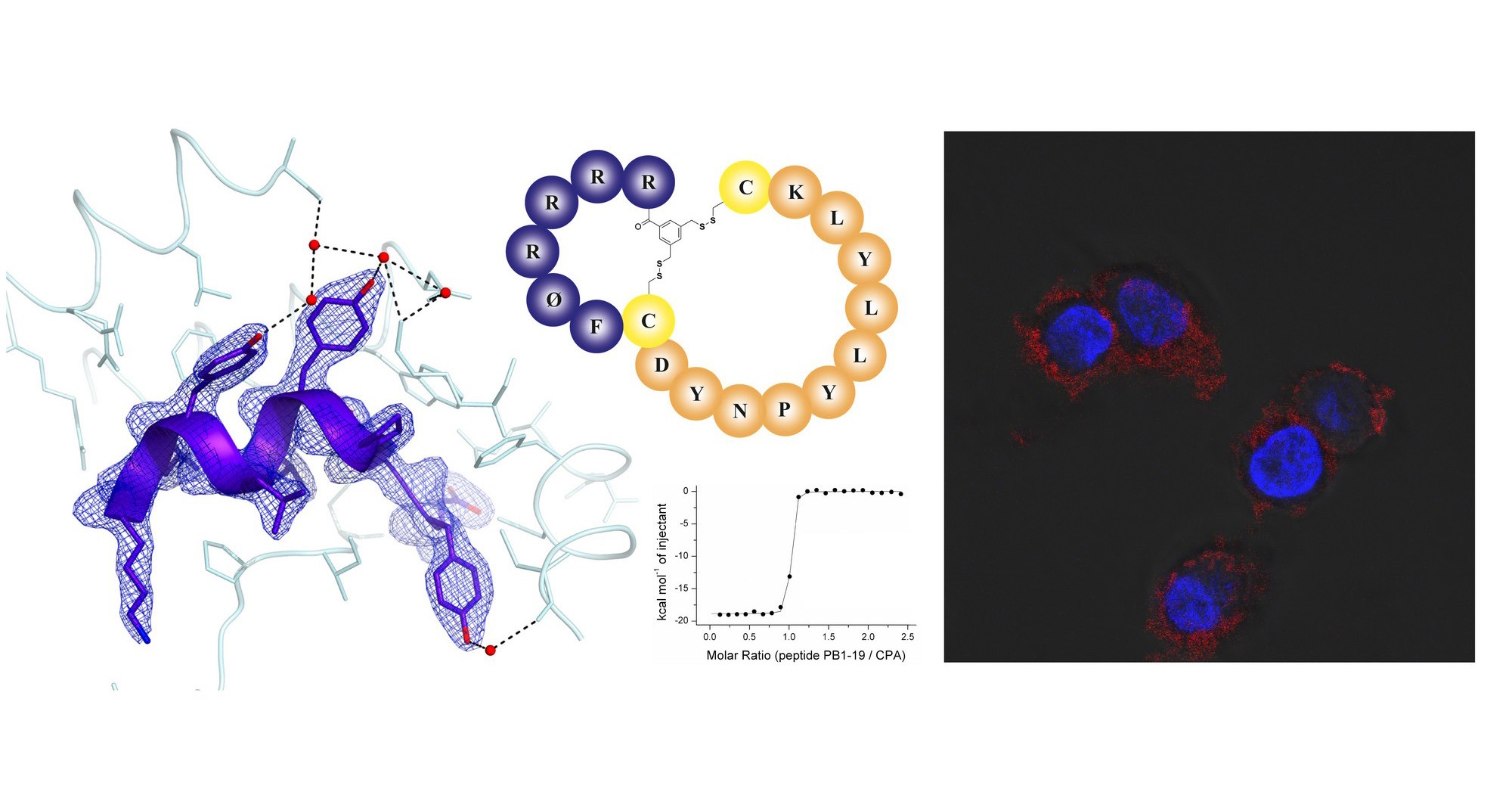
The study also covers the thermodynamic and structural characterization of the interaction between the PA subunit of the polymerase and the inhibitor. Solved the X-ray structure of the peptide-PA complex provided the researchers important structural insights into the interaction.
The crowning achievement was an improved proteolytic stability and endosomal escape of the inhibitor accomplished by application of cutting-edge reversible bicyclization strategy. This scientific work paves the way for the future development of more effective anti-influenza therapy.
Original article: Radilová, K.; Zima, V.; Kráľ, M.; Machara, A.*; Majer, P.; Hodek, J.; Weber, J.; Brynda, J.; Strmeň, T.; Konvalinka, J.; Kožíšek, M.* Thermodynamic and structural characterization of an optimized peptide-based inhibitor of the influenza polymerase PA-PB1 subunit interaction. Antiviral Res. 2022, 208, 105449. https://doi.org/10.1016/j.antiviral.2022.105449