
Prof. Alan R. Katritzky (University of Florida, Gainesville, FL, USA)
Novel N-, O-, S-, and C-Acylations and Related Reactions. Peptides and More
Abstrakt
Acylbenzotriazoles are advantageously prepared directly from the corresponding carboxylic acids by two routes which enable the easy preparation of RCOBt of which the corresponding acyl chlorides are difficult to prepare, unstable, hydroscopic, and/or require special handling. Thus α,β-olephinic and α,β-acetylinic carboxylic acids, heterocyclic derivatives including pyridines, furans, indoles, and hydroxy acids all give RCOBt in high yields: nearly all are RCOBt are crystalline.
N- α-(Protected amino)acylbenzotriazoles 1 have been prepared for 17 of the 20 natural amino acids. Only the amino functionality needs to be protected in serine, tyrosine, tryptophan, methionine, and cysteine. Compounds 1 are stable, crystalline (mp 100-200 ºC) and soluble in organic solvents. They are nonhydrosophic and can be handled and weighted without special precautions.
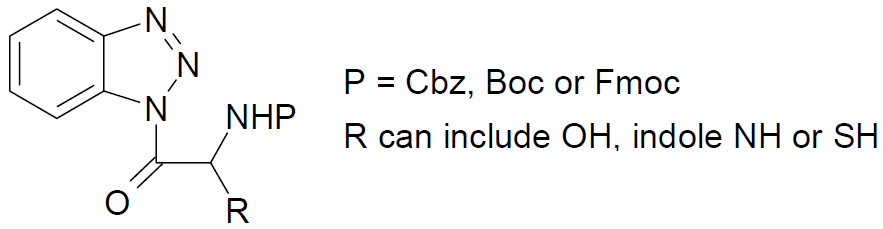
Acylbenzotriazoles, including 1, are excellent N-acylating agents. Importantly they can be used in partially aqueous solutions, which enable the acylation of amino acids without the need to prepare esters soluble in organic solvents. Di-, tri-, and tetra-peptides have thus been prepared.
Acylbenzotriazoles are also efficient agents for O- and S- acylation reactions which occur in high yield. α-Aminoacyl derivatives of terpenes, lipids, and steroids will be reported. C-Acylation by RCOBt allows the efficient preparation of a wide variety of bifunctional compounds.
Compounds XN=CBt2 and analogs enable a wide range of efficient imidoylations, providing high-yielding routes to amidines and guanidines.








