
Prof. Peter R. Schreiner (Justus-Liebig University Giessen, Německo)
Noncovalent Organocatalysis
Abstrakt
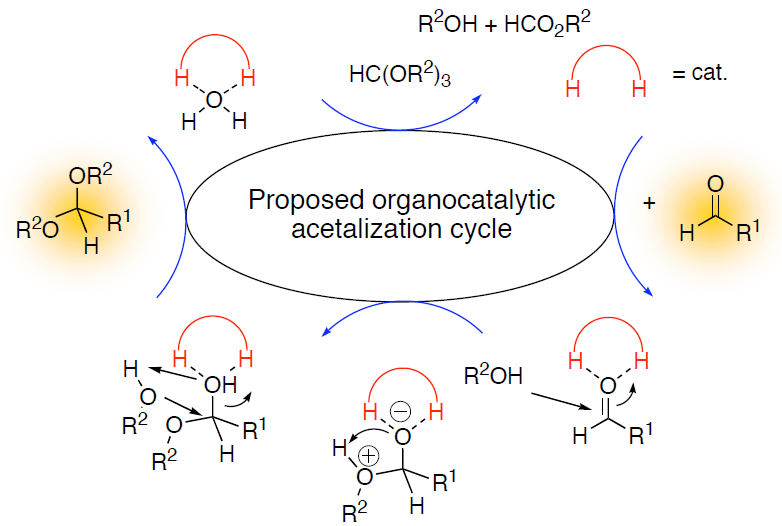
Organocatalysis combines the concepts of molecular recognition and supramolecular chemistry with enzyme-like catalytic concepts; this branch of research may therefore be coined with the notion of “the hunt for the smallest enzyme.”1 Noting that about half of all enzymes do not carry a metal center it is obvious that this approach has long been underappreciated. Although organocatalysis as such is a new field, it is already possible to catalyze many types of organic reactions with small, well-designed organic molecules. This circumvents the use of often toxic metals, and the preparation of the catalysts is much easier as it relies on the well-developed synthetic arsenal for tailor-making organic structures. While many approaches rely on covalent attachment of the catalyst (e.g., proline catalysis), we focus entirely on non-covalently bound catalysts;2 the talk will emphasize the notion of noncovalent bonding that is in line with Pauling’s paradigms of enzyme activity. In our group we have developed hydrogen-bonding thiourea-based catalysts that are effective in catalyzing Diels-Alder reactions,2 acid-free acetalizations,3 epoxide openings,4 and other reactions. Many other groups have not picked up this catalytic principle and apply it to a manifold of fascinating reactions.
Phase-transfer catalysis (PTC) is a special case of organocatalysis that has been around for several decades; PTC has enormous industrial applications. Our aim was to develop completely novel PTC reactions, for instance, metal-free PTC radical reactions for the direct halogenation of unactivated hydrocarbons.5 This shows the power of the organocatalytic approach as some of these reactions are not even feasible (e.g., iodination) with their organometallic counterparts.
Zdroje
- Schreiner, P. R. Chem. Soc. Rev. 2003, 32, 289-296.
- Wittkopp, A.; Schreiner, P. R. In The Chemistry of Dienes and Polyenes; Rappoport, Z., Ed.; John Wiley & Sons: Chichester, 2000; Vol. 2, pp 1029-1088; Schreiner, P. R.; Wittkopp, A. Org. Lett. 2002, 4, 217-220; Wittkopp, A.; Schreiner, P. R. Chem. Eur. J. 2003, 9, 407-414.
- Kotke, M.; Schreiner, P. R. Tetrahedron 2005, in print.
- Kleiner, C.; Schreiner, P. R., in preparation.
- Schreiner, P. R.; Fokin, A. A.; Lauenstein, O.; Okamoto, Y.; Wakita, T.; Rinderspacher, C.; Robinson, G. H.; Vohs, J. K.; Campana, C. F. J. Am. Chem. Soc. 2002, 124, 13348-13349; Schreiner, P. R.; Lauenstein, O.; Butova, E. D.; Gunchenko, P. A.; Kolomitsin, I. V.; Wittkopp, A.; Feder, G.; Fokin, A. A. Chem. Eur. J. 2001, 7, 4996–5003; Fokin, A. A.; Lauenstein, O.; Gunchenko, P. A.; Schreiner, P. R. J. Am. Chem. Soc. 2001, 123, 1842-1847; Lauenstein, O.; Fokin, A. A.; Schreiner, P. R. Org. Lett. 2000, 2, 2201-2204; Schreiner, P. R.; Lauenstein, O.; Butova, E. D.; Fokin, A. A. Angew. Chem. Int. Ed. Engl. 1999, 38, 2786-2788; Schreiner, P. R.; Lauenstein, O.; Kolomitsyn, I. V.; Nadi, S.; Fokin, A. A. Angew. Chem. Int. Ed. Engl. 1998, 37, 1895-1897.







